Organic Chemistry is a dynamic and ever-evolving discipline that serves as the cornerstone of numerous scientific and industrial advancements. It holds immense significance for students preparing for competitive exams such as IIT-JEE, NEET, and CSIR-NET, as well as professionals working in sectors like pharmaceuticals, petrochemicals, material science, and agrochemicals. At heart of this vast subject lie name reactions those pivotal chemical transformations named after the chemists who first discovered or developed them. These reactions are not only academically important but are also foundational to practical organic synthesis in real-world applications.
This comprehensive blog dives into the fascinating world of name reactions, exploring their detailed mechanisms, underlying principles, and practical applications. Whether, you’re a curious student, an exam aspirant, or a passionate chemist, this in-depth guide will enhance your understanding and appreciation of these classic transformations.
What Are Name Reactions?
Name reactions are well-established organic reactions that have been given names-often honoring their discoverers. These reactions are essential because they represent standard and reliable methods to construct complex molecules from simpler ones. They offer a systematic approach to achieving chemical synthesis, particularly in multi-step reaction sequences.
Importance of Learning Name Reactions
- Predictability: These reactions follow established mechanisms, making it easier to forecast products and intermediates.
- Reproducibility: Due to their well-documented nature, name reactions are repeatable under defined conditions.
- Exam Relevance: Frequently asked in academic exams like IIT-JEE, NEET, CSIR-NET, GATE, and UPSC.
- Industrial Relevance: Widely used in the manufacture of dyes, drugs, agrochemicals, perfumes, and plastics.
- Foundation for Advanced Study: Understanding these reactions lays the groundwork for learning more complex synthetic strategies.
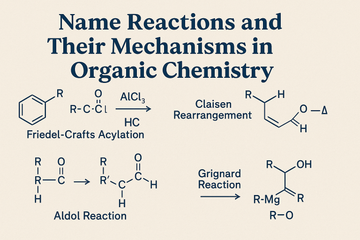
1. Aldol Condensation
Reaction Type: Carbon–Carbon bond formation
Mechanism:
- Base abstracts an α-hydrogen to form an enolate ion.
- The enolate attacks the carbonyl carbon of another molecule.
- Formation of β-hydroxy aldehyde or ketone.
- Dehydration under heat to yield α,β-unsaturated carbonyl compound.
Applications:
- Vital in the synthesis of polyenes, fragrances, and pharmacologically active molecules.
- Often used in developing building blocks for polymers.
2. Cannizzaro Reaction
Reaction Type: Redox reaction for aldehydes without α-hydrogens
Mechanism:
- Base attacks a non-enolizable aldehyde forming a tetrahedral intermediate.
- One molecule gets oxidized to carboxylate, while the other is reduced to alcohol.
Applications:
- Industrial production of benzyl alcohol.
- Synthesis of aromatic alcohols and acids without using external oxidizing/reducing agents.
3. Friedel–Crafts Alkylation and Acylation
Reaction Type: Electrophilic aromatic substitution
Mechanism:
- Alkylation: Alkyl halide forms carbocation with AlCl₃; carbocation acts as electrophile.
- Acylation: Acid chloride forms acylium ion, which reacts with aromatic ring.
Applications:
- Introduction of alkyl and acyl groups into aromatic systems.
- Synthesis of pharmaceuticals, perfumes, and agrochemicals.
4. Grignard Reaction
Reaction Type: Nucleophilic addition
Mechanism:
- Organomagnesium compound (Grignard reagent) attacks electrophilic carbon in carbonyl.
- Addition intermediate formed; hydrolysis gives alcohol.
Applications:
- Preparation of secondary and tertiary alcohols.
- Backbone for making alcohols, carboxylic acids, and hydrocarbons.
5. Diels–Alder Reaction
Reaction Type: [4+2] Cycloaddition
Mechanism:
- Diene and dienophile react via concerted mechanism.
- Formation of six-membered cyclic compound.
Applications:
- Central in total synthesis of natural products and pharmaceuticals.
- Used in polymer chemistry and in designing molecular switches.
6. Wurtz Reaction
Reaction Type: Coupling reaction
Mechanism:
- Alkyl halide reacts with sodium metal in dry ether.
- Formation of free radicals; combination results in C–C bond.
Applications:
- Useful for making symmetrical alkanes.
- Historically significant in organic synthesis of hydrocarbons.
7. Sandmeyer Reaction
Reaction Type: Nucleophilic substitution using diazonium salts
Mechanism:
- Primary aromatic amine is converted to diazonium salt.
- Salt is reacted with CuX (X = Cl, Br, CN) to substitute the diazonium group.
Applications:
- Introduction of functional groups into benzene rings.
- Essential in dye chemistry, pharmaceuticals, and agrochemicals.
8. Reimer–Tiemann Reaction
Reaction Type: Electrophilic substitution using dichlorocarbene
Mechanism:
- Phenol reacts with chloroform in basic medium.
- Dichlorocarbene intermediate forms; ortho-formylation of phenol occurs.
Applications:
- Synthesis of salicylaldehyde, an important intermediate in fragrances and antiseptics.
9. Kolbe’s Reaction
Reaction Type: Carboxylation reaction
Mechanism:
- Sodium phenoxide reacts with carbon dioxide under pressure.
- Electrophilic attack on ortho position; yields salicylic acid upon acidification.
Applications:
- Industrial production of aspirin and other salicylates.
10. Beckmann Rearrangement
Reaction Type: Rearrangement of oximes
Mechanism:
- Oxime is treated with acid catalyst.
- Rearrangement to give amide by migration of the R-group.
Applications:
- Key step in production of caprolactam, used in the manufacture of nylon-6.
11. Hofmann Bromamide Reaction
Reaction Type: Conversion of amides to amines
Mechanism:
- Amide is treated with bromine and aqueous base.
- Alkyl group migrates, CO₂ is eliminated; results in primary amine.
Applications:
- Shortening of carbon chains.
- Synthesis of aromatic and aliphatic amines.
12. Hell–Volhard–Zelinsky Reaction
Reaction Type: α-Halogenation of carboxylic acids
Mechanism:
- Carboxylic acid is converted to acyl bromide.
- Bromination occurs at α-position.
Applications:
- Preparation of α-halo acids.
- Used in synthesis of amino acids and pharmaceutical intermediates.
13. Perkin Reaction
Reaction Type: Aldol-like condensation
Mechanism:
- Aromatic aldehyde reacts with an acid anhydride in the presence of sodium salt.
- Forms α,β-unsaturated aromatic acid.
Applications:
- Synthesis of cinnamic acid derivatives.
- Important in perfume and flavoring industries.
14. Claisen Condensation
Reaction Type: Ester condensation
Mechanism:
- Ester is deprotonated to form enolate.
- Enolate attacks carbonyl carbon of another ester.
- Forms β-keto ester upon elimination.
Applications:
- Synthesis of β-dicarbonyl compounds.
- Key step in the manufacture of barbiturates and other drugs.
15. Wolf–Kishner Reduction
Reaction Type: Conversion of carbonyls to methylene groups
Mechanism:
- Carbonyl compound reacts with hydrazine to form hydrazone.
- Heating with strong base removes nitrogen; reduces to alkane.
Applications:
- Used to reduce carbonyl groups without affecting other functional groups.
- Preferred in high-temperature conditions where metal catalysts are avoided.
Effective Strategies to Master Name Reactions
- Understand the Logic: Focus on electron movement, not just product memorization.
- Visual Learning: Use diagrams and animations to visualize mechanisms.
- Flashcard Practice: Regularly test yourself using reaction cards.
- Group Study: Discussing reactions with peers helps reinforce concepts.
- Real-World Connections: Explore how reactions are applied in industry and drug synthesis.
Frequently Asked Examination Formats
- Fill-in-the-blank mechanisms.
- MCQs on reagents, intermediates, and products.
- Naming reactions based on conditions and products.
- Identifying rearrangements and transition states.
Mastering name reactions is not just about rote learning—it's about developing a deep understanding of how and why molecules transform. These reactions are invaluable tools in organic chemistry, providing the blueprint for creating new compounds, studying reaction pathways, and solving real-world challenges in drug discovery, materials science, and chemical manufacturing.
Keep practicing mechanisms, stay curious, and appreciate the elegance of organic transformations. This knowledge will serve as your foundation, not only for academic success but also for innovative problem-solving in chemical research and industry.







6bpx4k
69vidh
kr828u